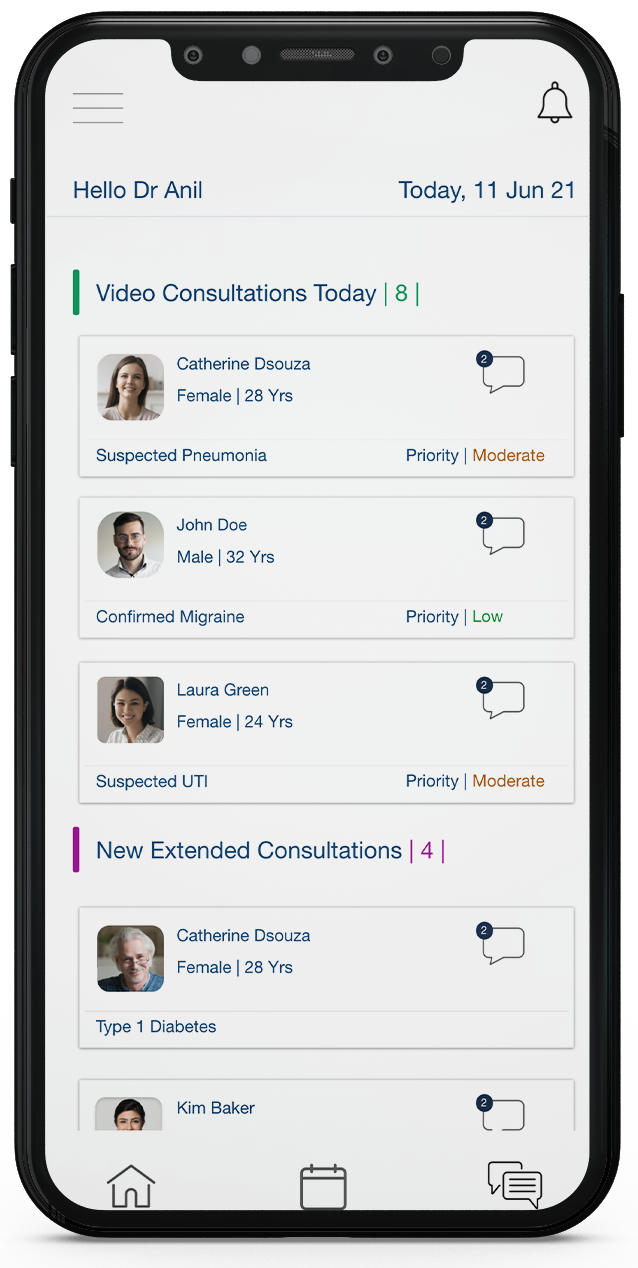
Our unique virtual hospital solution allows virtual team building by doctors and researchers across the nation. This allows the team to synchronize the use of Dockares’ remote patient monitoring, symptom based alert and configurable e-CRF to measure real world evidence of various therapeutic areas.

Our unique virtual hospital concept allows virtual team building by doctors and researchers across the nation. This allows the team to synchronize the use of Dockares’ remote patient monitoring, symptom based alert and configurable e-CRF to measure real world evidence of various therapeutic areas.

Replicate the real-hospital scenario with superior patient- doctor engagement and structured care-giver network all under one roof. Creating a research team on Dockare has never been so easy.

Data collected digitally at source, with no manual transcription and hand-offs coupled with intelligent validations drive data to be of high quality and reliability. Increases the quality of insight generation. Stage-gated quality check using advanced ML algorithms help achieve high levels of specificity and accuracy.

Transform your study objectives and clinical endpoints to intuitive e-CRFs. Our e-CRFs are easily created and validated for accurate data collection and effective execution of RWE studies. Pick from out-of-the box templates using a wizard directed across purpose and therapeutic areas to quickly deploy these forms.

Our platform is designed on global standards of HL7 and FHR data structure making you ready for standard data import and export. Widening your data architecture across EHRs, clinical and multiple other disparate patient data sources making it wider, deeper and richer for generating high quality insights.

Dockares’ data structure is well-organized, making analysis less cumbersome, reducing cleaning efforts and providing our data scientists to look farther into outcomes. Dockares’ market-ready analytic plan allows you to reach insights faster and solving your complex business challenges with ease.

Over 100+ years of collective experience of our cross-functional team across clinical, hospital, regulatory, payer HTA assessors and clinical scientists makes Dockare to transform your strategies to reality by providing pointed insights and specific evidences to fulfil your business objectives.
Think beyond regulatory, clinical and development to collect relevant real-world evidence from unrestricted access to millions of anonymized patient records to power your commercialization efforts. Partner with our consulting team to determine the most cost-effective methodology to accelerate product approval, enhance prescriptions and market access through our services:
